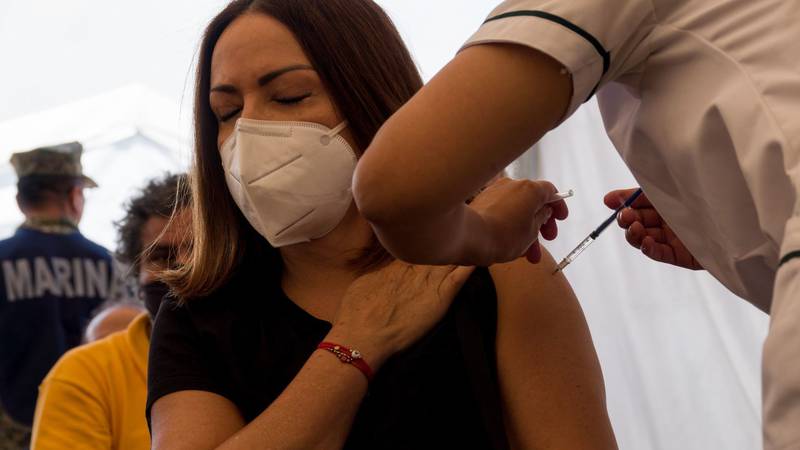
MEXICO CTY, Nov 21 (NNN-PRENSA LATINA) — Mexico’s Federal Commission for Protection against Sanitary Risks (COFEPRIS) on Sunday authorized Cuba’s Soberana and Soberana Plus homegrown vaccines for emergency use in adults.
In a statement, COFEPRIS explained that they have distinctive names: recombinant protein of the receptor binding domain of the SARS-CoV-2 virus (RBD) conjugated to tetanus toxoid; and recombinant SARS-CoV-2 virus receptor binding domain (RBD) protein.
It adds that the authorizations issued by this commission are part of the National Health Regulation Strategy, which allows reviewing and giving access to the largest number of healthcare supplies, as long as the quality, safety, and efficacy of the product are verified.
As a National Regulatory Authority of reference qualified by the Pan-American Health Organization (WHO), COFEPRIS decisions are recognized by various countries of the continent, so the approved vaccines are likely to be used in other nations.
It pointed out that being a member of the International Conference on Harmonization of Technical Requirements for the Registration of Pharmaceutical Products for Human Use, known as ICH, all decisions by this authority are based on evidence technical-scientific presented.
It indicated that the Cuban vaccines received a favorable opinion from the National Committee for Science and Technology and Innovation in Health of the National Council of Science and Technology on Nov 26, 2021.
Subsequently, the New Molecules Committee (CMN) met on Sept 9, 2022, to discuss the use of Cuba’s vaccines, which received a favorable technical opinion from the experts.
After integrating the opinion of the CMN and submitting the authorization request for emergency use to COFEPRIS, personnel specialized in vaccines analyzed the files, certifying that the vaccines meet the standards of quality, safety, and efficacy necessary to be administers, it added. — NNN-PRENSA LATINA